Sulphuric Acid Titration With Sodium Hydroxide . Titration of sulphuric acid with sodium hydroxide modified: Introductory chemistry demonstrations in the laboratory. Sulfuric acid is titrated against sodium. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. November 3, 2002 introduction a titration is. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. \ce{naoh}\) is required to neutralize. In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it.
from www.youtube.com
Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. Introductory chemistry demonstrations in the laboratory. \ce{naoh}\) is required to neutralize. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Titration of sulphuric acid with sodium hydroxide modified: November 3, 2002 introduction a titration is. Sulfuric acid is titrated against sodium. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it.
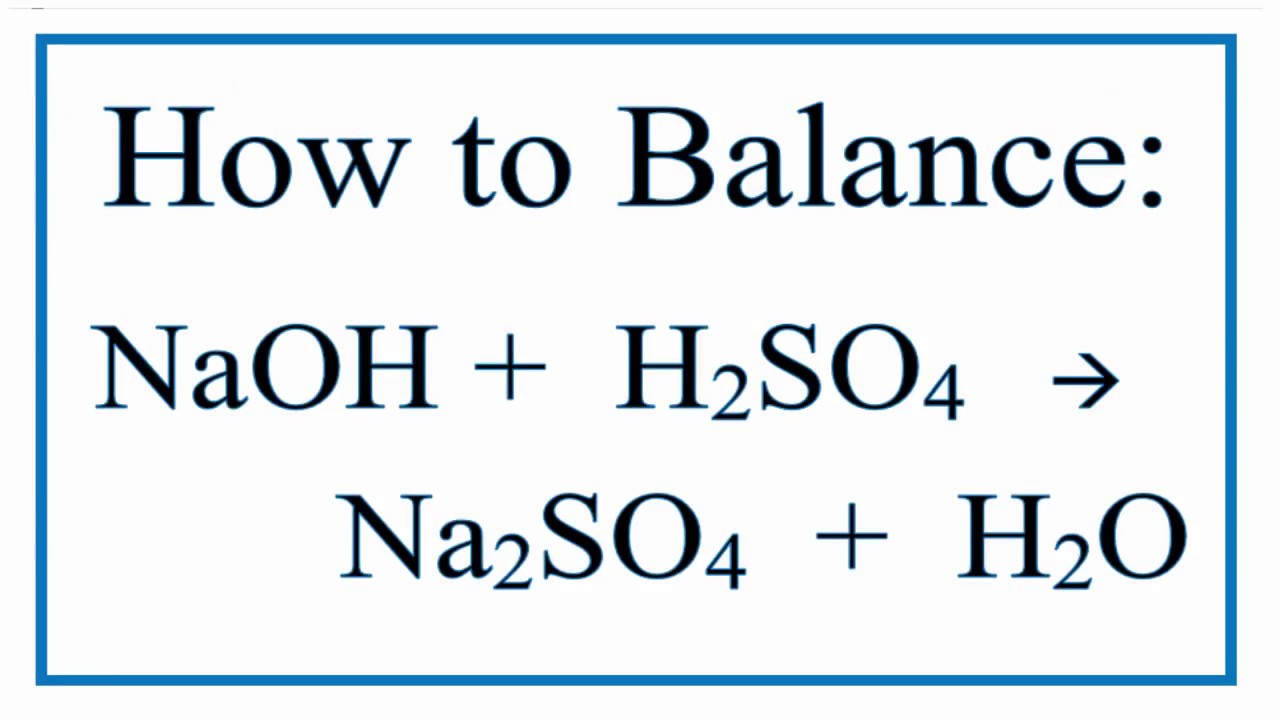
Sodium Hydroxide and Sulfuric Acid yields Sodium sulfate and Water
Sulphuric Acid Titration With Sodium Hydroxide In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. Introductory chemistry demonstrations in the laboratory. \ce{naoh}\) is required to neutralize. Sulfuric acid is titrated against sodium. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Titration of sulphuric acid with sodium hydroxide modified: November 3, 2002 introduction a titration is.
From www.myxxgirl.com
Sulphuric Acid Sodium Hydroxide My XXX Hot Girl Sulphuric Acid Titration With Sodium Hydroxide Sulfuric acid is titrated against sodium. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it. In. Sulphuric Acid Titration With Sodium Hydroxide.
From www.hotixsexy.com
Naoh H2so4 Free Nude Porn Photos Sulphuric Acid Titration With Sodium Hydroxide In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. In a titration of sulfuric acid against sodium. Sulphuric Acid Titration With Sodium Hydroxide.
From www.chegg.com
Titration of sulfuric acid Average Volume of NaOH = Sulphuric Acid Titration With Sodium Hydroxide Introductory chemistry demonstrations in the laboratory. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. \ce{naoh}\) is. Sulphuric Acid Titration With Sodium Hydroxide.
From www.youtube.com
Sodium Hydroxide and Sulfuric Acid YouTube Sulphuric Acid Titration With Sodium Hydroxide Titration of sulphuric acid with sodium hydroxide modified: In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. \ce{naoh}\) is required to neutralize. Sulfuric acid is titrated against sodium. Introductory chemistry demonstrations in the laboratory. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: That. Sulphuric Acid Titration With Sodium Hydroxide.
From www.youtube.com
Sodium Hydroxide + Sulfuric Acid Acid Base Neutralization Reaction Sulphuric Acid Titration With Sodium Hydroxide In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Titration of sulphuric acid with sodium hydroxide modified: Sulfuric acid is titrated against sodium. \ce{naoh}\) is required to neutralize. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide. Sulphuric Acid Titration With Sodium Hydroxide.
From fity.club
Titration Of Hcl With Naoh Sulphuric Acid Titration With Sodium Hydroxide November 3, 2002 introduction a titration is. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. Titration of sulphuric acid with sodium hydroxide modified: That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. Introductory chemistry demonstrations in the laboratory. In a titration of sulfuric acid against sodium hydroxide, \(32.20. Sulphuric Acid Titration With Sodium Hydroxide.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Sulphuric Acid Titration With Sodium Hydroxide Introductory chemistry demonstrations in the laboratory. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it. Titration of sulphuric acid with sodium hydroxide modified: November 3, 2002 introduction a titration is. Sulfuric acid reacts with sodium. Sulphuric Acid Titration With Sodium Hydroxide.
From www.toppr.com
12 mL of 0.25 N sulphuric acid is neutralised with 15 mL of sodium Sulphuric Acid Titration With Sodium Hydroxide In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Introductory chemistry demonstrations in the laboratory. In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it. \ce{naoh}\) is required to neutralize. In a titration. Sulphuric Acid Titration With Sodium Hydroxide.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Sulphuric Acid Titration With Sodium Hydroxide In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is titrated against sodium. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. November 3,. Sulphuric Acid Titration With Sodium Hydroxide.
From ar.inspiredpencil.com
Sulfuric Acid And Sodium Hydroxide Sulphuric Acid Titration With Sodium Hydroxide In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it. Titration of sulphuric acid with sodium hydroxide modified: \ce{naoh}\) is required to neutralize. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. Introductory chemistry demonstrations. Sulphuric Acid Titration With Sodium Hydroxide.
From syatillakmk.blogspot.com
SimplyChemistry TITRATION OF SULFURIC ACID WITH SODIUM HYDROXIDE Sulphuric Acid Titration With Sodium Hydroxide November 3, 2002 introduction a titration is. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. Sulfuric acid is titrated against sodium. Sulfuric acid reacts with sodium. Sulphuric Acid Titration With Sodium Hydroxide.
From teachernotes4u.com
Diagram shows the apparatus setup to study the reaction between sodium Sulphuric Acid Titration With Sodium Hydroxide That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Sulfuric acid is titrated against sodium. Introductory chemistry demonstrations in the laboratory. Titration of sulphuric acid with sodium hydroxide modified: In reality, this is a stoichiometric question where you are. Sulphuric Acid Titration With Sodium Hydroxide.
From www.yumpu.com
titration of sulphuric acid with sodium hydroxide sentinelchem Sulphuric Acid Titration With Sodium Hydroxide Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. Sulfuric acid is titrated against sodium. Titration of sulphuric acid with sodium hydroxide modified: Introductory chemistry demonstrations in the laboratory. In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it. \ce{naoh}\) is required to neutralize. That means. Sulphuric Acid Titration With Sodium Hydroxide.
From www.sarthaks.com
In an experiment to determine the enthalpy of neutralisation of sodium Sulphuric Acid Titration With Sodium Hydroxide That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: \ce{naoh}\) is required to neutralize. November 3, 2002 introduction a titration is. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it. Sulphuric Acid Titration With Sodium Hydroxide.
From www.docsity.com
TITRATION OF SULPHURIC ACID WITH SODIUM HYDROXIDE Slides Chemistry Sulphuric Acid Titration With Sodium Hydroxide Sulfuric acid is titrated against sodium. Titration of sulphuric acid with sodium hydroxide modified: That means number of moles of sulfuric acid is half that of number of moles of sodium hydroxide used. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: November 3, 2002 introduction a titration is. In my latest chem lab the objective was to. Sulphuric Acid Titration With Sodium Hydroxide.
From www.scribd.com
Titration of Sulphuric Acid With Sodium Hydroxide PDF Chemistry Sulphuric Acid Titration With Sodium Hydroxide November 3, 2002 introduction a titration is. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: \ce{naoh}\) is required to neutralize. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is titrated against sodium. In reality, this is a stoichiometric question where. Sulphuric Acid Titration With Sodium Hydroxide.
From www.slideserve.com
PPT Transition Metals (Cr, Mn , Fe, Co, Ni and Cu) PowerPoint Sulphuric Acid Titration With Sodium Hydroxide \ce{naoh}\) is required to neutralize. Titration of sulphuric acid with sodium hydroxide modified: Introductory chemistry demonstrations in the laboratory. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid is titrated against sodium. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. In reality,. Sulphuric Acid Titration With Sodium Hydroxide.
From www.meritnation.com
Sulphuric acid react with sodium hydroxide as follows when 1L of 0 1M Sulphuric Acid Titration With Sodium Hydroxide \ce{naoh}\) is required to neutralize. In reality, this is a stoichiometric question where you are trying to find the molarity of an unknown polyprotic acid by titrating it. Sulfuric acid reacts with sodium hydroxide on the 1:2 basis. November 3, 2002 introduction a titration is. Titration of sulphuric acid with sodium hydroxide modified: Introductory chemistry demonstrations in the laboratory. That. Sulphuric Acid Titration With Sodium Hydroxide.